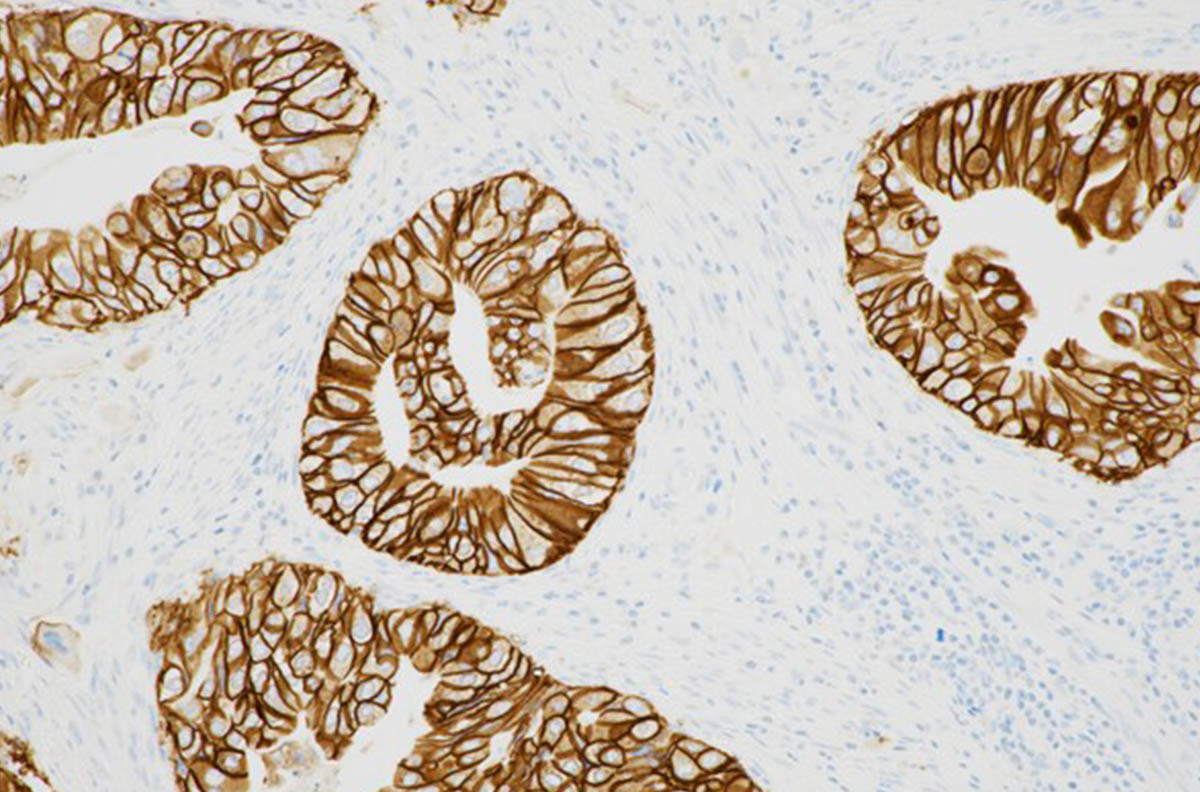
Roche Gets Key FDA Approval for Companion Diagnostic in Biliary Tract Cancer
Roche has announced U.S. Food and Drug Administration approval of a label expansion into biliary tract cancer for the PATHWAY® anti-HER2/neu (4B5) Rabbit Monoclonal Primary Antibody* test. This test is now the first and only FDA-approved companion diagnostic to aid in the assessment of HER2-positive status to identify BTC patients who are eligible for treatment with Jazz Pharmaceuticals’ ZIIHERA® (zanidatamab-hrii).
HER2 is a receptor protein expressed in a variety of cancers and serves as a predictive biomarker to help determine if a patient will respond to HER2-targeted therapy.1 No approved and validated HER2 test existed to identify eligible BTC patients until the approval of this expanded label for the PATHWAY HER2 (4B5) test.
“This test is a step forward in furthering access to personalized medicine,” said Jill German, head of pathology lab at Roche Diagnostics. “The prognosis for patients diagnosed with BTC is poor, as very few treatment options exist. Now, these patients have access to the first standardized test that could make them eligible for targeted therapy, potentially improving clinical outcomes.”
ZIIHERA is the first FDA-approved treatment for adults with previously treated, unresectable or metastatic HER2-positive (IHC 3+) biliary tract cancer.
BTC accounts for 3% of all gastrointestinal cancers in the U.S.Prognosis is poor because of a lack of adequate early detection tools, challenging anatomical access, aggressive tumor biology, and only modest benefit from systemic treatments.With most cases diagnosed at an advanced stage,the five-year overall survival rate for all BTC cases is 19% for disease that is localized to the original tumor site, and just 3% for cancer that has spread to other areas.





